How to Outsource Clinical Trials
Drug development is one of the most competitive industries in the world. Pharmaceutical and biotech companies work non-stop to find ways to optimize product development and efficiency while staying affordable.
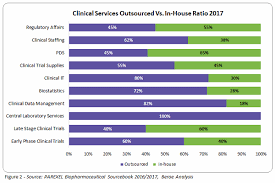
Outsourcing aspects of the clinical trial or drug development process to international teams has been established as an effective method of saving time and resources. Outsourcing also offers pharma and biotech companies access to larger and more diverse patient pools. The process of outsourcing clinical trials is, however, very complex. Regulating standards and achieving effective results requires clear channels of communication and the kind of expert clinical trial management provided by clinical trial companies like Client Pharma.
In this article, we will present an overview of clinical trial outsourcing and the main challenges facing the market today.
Table of contents:
- What factors have led to increased outsourcing of clinical trials?
- Main challenges when outsourcing clinical trials
What factors have led to increased outsourcing of clinical trials?
Beginning in the 1970s, outsourcing was largely limited to a few big pharmaceutical companies. But over the years, with the introduction of third-party vendors, the costs associated with clinical trial outsourcing came down. Now outsourcing clinical trials is a viable option for smaller biopharma companies as well.
Two of the most important drivers of the increase in clinical trial outsourcing are—the need for efficiency and reduction in research and development costs. By outsourcing clinical trials, a pharmaceutical company can use the client organization’s therapeutic and operational expertise, geographic reach, processes, and tool, while keeping their costs to the minimum. Companies like Client Pharma specialize in providing such clinical trial management services.
Outsourcing has also made it possible for research in specialized therapeutic areas, for example, oncology, to be carried out by small- and medium-sized organizations which would otherwise not have the expertise or resources to develop related products.
Main challenges when outsourcing clinical trials
The need for accurate translation: Trial protocols differ from one country to another, just as they differ from one company to another. With the globalization of clinical research, trial protocols are often designed in one country but implemented in another. This gives rise to the need for highly accurate translation services at almost every stage of the clinical trial process.
In addition to the complex trial protocol, translators are also required to write up brochures for the researchers, patient information documents, legal agreements, industry-specific jargon, etc.
Increasing protocol design complexity: The increasing complexity of clinical trial protocols is leading to longer cycle times, impediments in recruiting and retaining volunteers for the study, and increases in protocol amendments (which are often unplanned, not budgeted for, and disruptive).
As the pressure to demonstrate the safety and efficacy of new treatments, as well as the cost-efficiency of developing them, increases, protocol complexity will likely continue to grow. Managing this increasing complexity will require greater expertise from CROs in the coming years, as well as greater communication and coordination with sponsors. Services that help manage CRO-sponsor relationships will be at a premium in the coming years.